Battery Life, Voltage, and How We Can Help
Everyone has someone in their life who will be sure to point out battery replacement costs when considering an electric vehicle. Are they right to be concerned?
Yes and no. Everyone considering an electric vehicle should know about its battery life, warranty, and possible replacement.
Cost: Batteries are expensive and electric vehicles have large battery packs. In 2016, on average the battery packs cost ~50% of the total vehicle, but with battery costs coming down this value has halved (Source). There is no perfect one to one comparison to conventional vehicles as batteries are essentially a very expensive gas tank, but for reference, the drivetrain and engine in a conventional vehicle account for 22-24% of the total cost (Source). Battery replacement costs (labor included) can vary widely from $10-30k - a cost no one wants to pay (Source).
Lifetime: Typical battery warranties for electric vehicle batteries expire after 8-10 years and/or 80-100k miles (Source). Among other symptoms, the warranty is triggered when the capacity/range falls below a certain percentage of its original, typically 70-80% (Source). Just because the warranty expires doesn’t mean the battery is useless. The battery performance varies with climate and use, but over the last decade, both expectations and technology have improved. A staggering 30% of 2011 model year EVs have had their batteries replaced, while just a year later that number dropped to 16% (Source). After a decade, <6% of 2014 model year EVs have had their battery replaced (Source).
Technology will continue to improve. Battery cost will continue to fall. While battery lifetime and replacement should remain a factor for anyone considering purchase of an EV, the situation has and will continue to improve. One note: we often hear Level 3 charging voids warranties. While faster charging does degrade the battery faster , we have not seen warranties that are voided by proper use of public Level 3 chargers. Users obviously need to check their specific documentation to be sure.
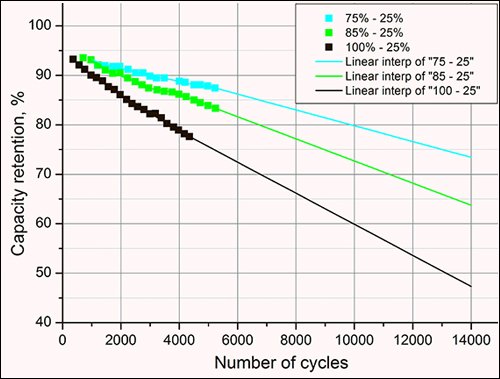
Warranty or not - we all want longer battery life. In our previous post, we noted that Li-ion batteries don’t like to linger in a charged state - also known as 100% state of charge (SOC). This is true of most battery chemistries and devices - cell phones, laptops, etc. Keen EV owners know that they should only charge their cars to 100% when necessary, and even then, it is recommended to use the built-in tools to time the end of charge as close to your road trip as possible (Source). Even if the cells don’t stay at 100% SOC for long, degradation still occurs. The figure below exemplifies this point, where limiting charging 75% SOC significantly enhances battery life - modeling a loss of more than 50% of original capacity when regularly charged to 100% SOC vs. only <30% loss when charging is limited to 75% SOC.
Battery University figure demonstrating battery life when charged to 75% SOC (blue, top), 85% SOC (green, middle), and 100% SOC (black, fastest capacity loss).
You might think: why not always charge to 75% - battery life problem solved! You would be partially correct. The amount of energy extracted from a cell is determined by the cell voltage. In his 2019 paper, Jeff Dahn - a leader in the field - and his team demonstrated lowering the SOC (energy extracted and therefore cell voltage) and optimizing the cathode and electrolyte produces a NMC532 cell with a lifetime comparable or better to LFP - historically considered the longest lived Li-ion battery chemistry. Again, in 2022 his team demonstrated a high-Ni cell with far greater life when cell voltage is lowered (see below).
Literature study demonstrating the strong impact of cell voltage (which controls the maximum state of charge - SOC).
Dark blue - higher energy/shorter life.
Light blue - lower energy/ longer life.
Pushing the Limits. Does this solve the lifetime challenge? No, but it tells us we have a choice between lifetime and energy. It also tells us that the target for advancements needs to focus on protecting against high voltage degradation. With consumers asking for longer range and higher energy, this will only get worse as the battery industry is moving toward higher voltages aiming to extract every bit of energy in the cell.
LiNK-BT partners with technology providers and invests in development of electrolyte components that stabilize this higher energy regime. Let’s review one example: vinylene carbonate (VC) - the traditional first choice for electrolyte additives.
We have previously discussed the solid electrolyte interphase (SEI) - the layer formed as the electrolyte decomposes on the electrode surface - serving as a stabilizer when the layer forms well. In the figure below, we see this as a small amount of electrochemical current/activity (red, VC) compared to the solvent alone (EC) around 3.75V. When the cell voltage increases that small activity grows out of control (right of the blue line). As a result, the SEI is thicker and worsens cell performance. This is one reason VC is ideal for one system and a poor choice in another. This is all the result of a switch from a single to double bond in the structure!
Assessment of vinylene carbonate (VC) showing the current (and therefore electrochemical activity) at increasing voltages. The blue line shows a typical cell voltage. As shown VC demonstrates far more activity at high voltages relative to the solvent (ethylene carbonate - EC).
At higher voltages we look to electrolyte components with enhanced voltage stability. This search has led to numerous papers and patents exploring different functional groups - which will continue to be both the topic of future posts and ongoing development work at LiNK-BT. Below we highlight Ascend Element’s Trinohex Ultra (1,3,6-hexanetricarbonitrile, H in the figure below) and tris(trimethylsilyl)phosphate - TTSP, T in the figure below). These linear sweep voltammograms (LSVs) demonstrate which voltages (x-axis) have electrochemical activity (current, y-axis). As shown, TTSP (T), while famous for other benefits, does NOT significantly extend voltage stability while H improve it. This highlights the need for diverse electrolyte components and product-specific electrolyte blends. LiNK-BT is always on the hunt for next generation additives and building a library of components to keep cell manufacturers at the cutting edge!
Linear sweep voltammograms (LSVs) depicting electrochemical activity (current) and different voltages in four electrolytes: a “basic electrolyte” LiPF6 in EC/DEC; and containing H (1,3,6-hexanetricarbonitrile) and T (tris(trimethylsilyl)phosphate), or both. We see here that electrolyte additives can drastically change the voltage stability of an electrolyte. Cell is a Li-rich MNC cathode vs. Li metal.
LiNK-BT is on the hunt. We partner with chemical manufacturers to develop our portfolio of advanced electrolyte components and screen existing products for suitability in electrolytes. We envision a world where consumers can have it all: range and lifetime, and we are investing in the development of the technology to make it happen.